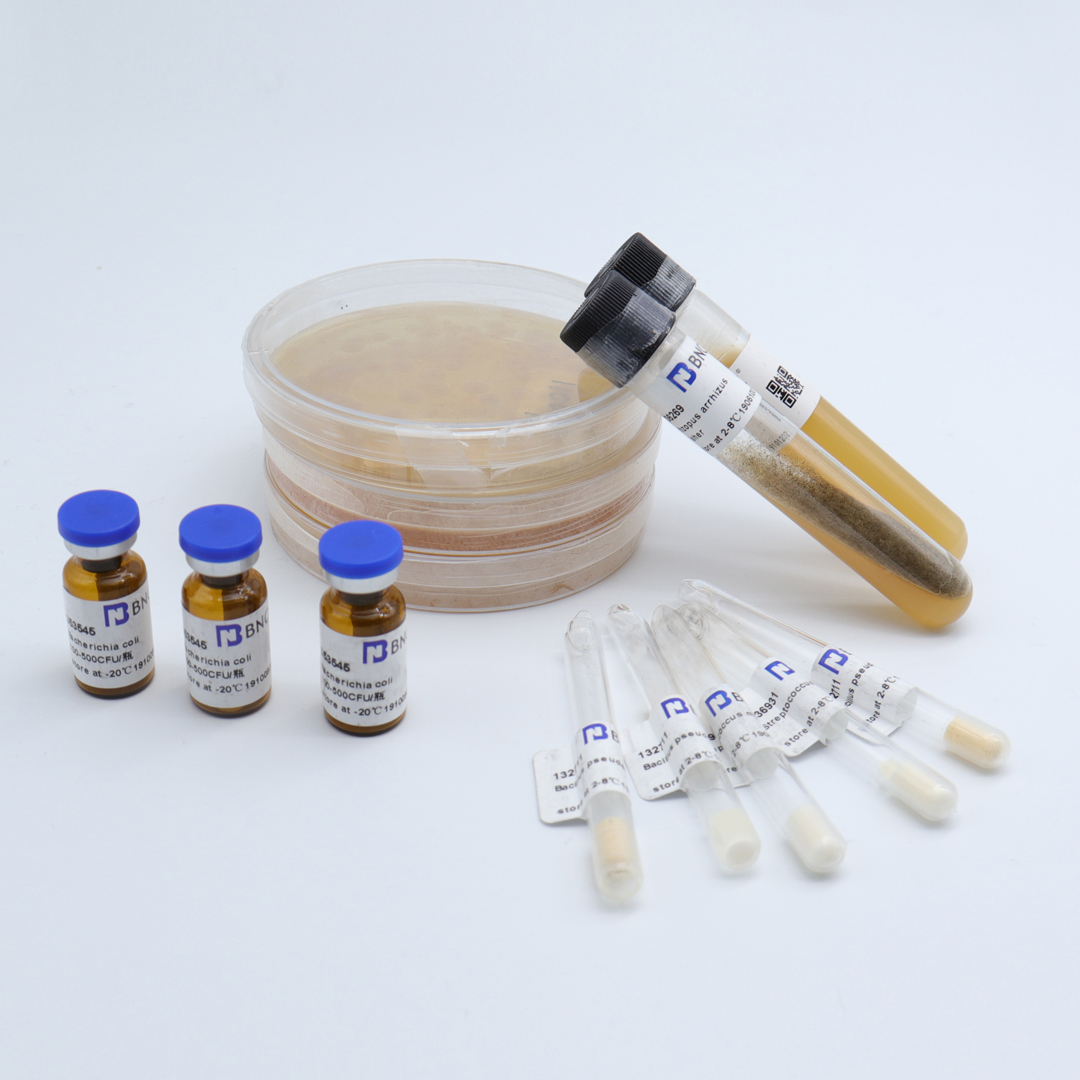
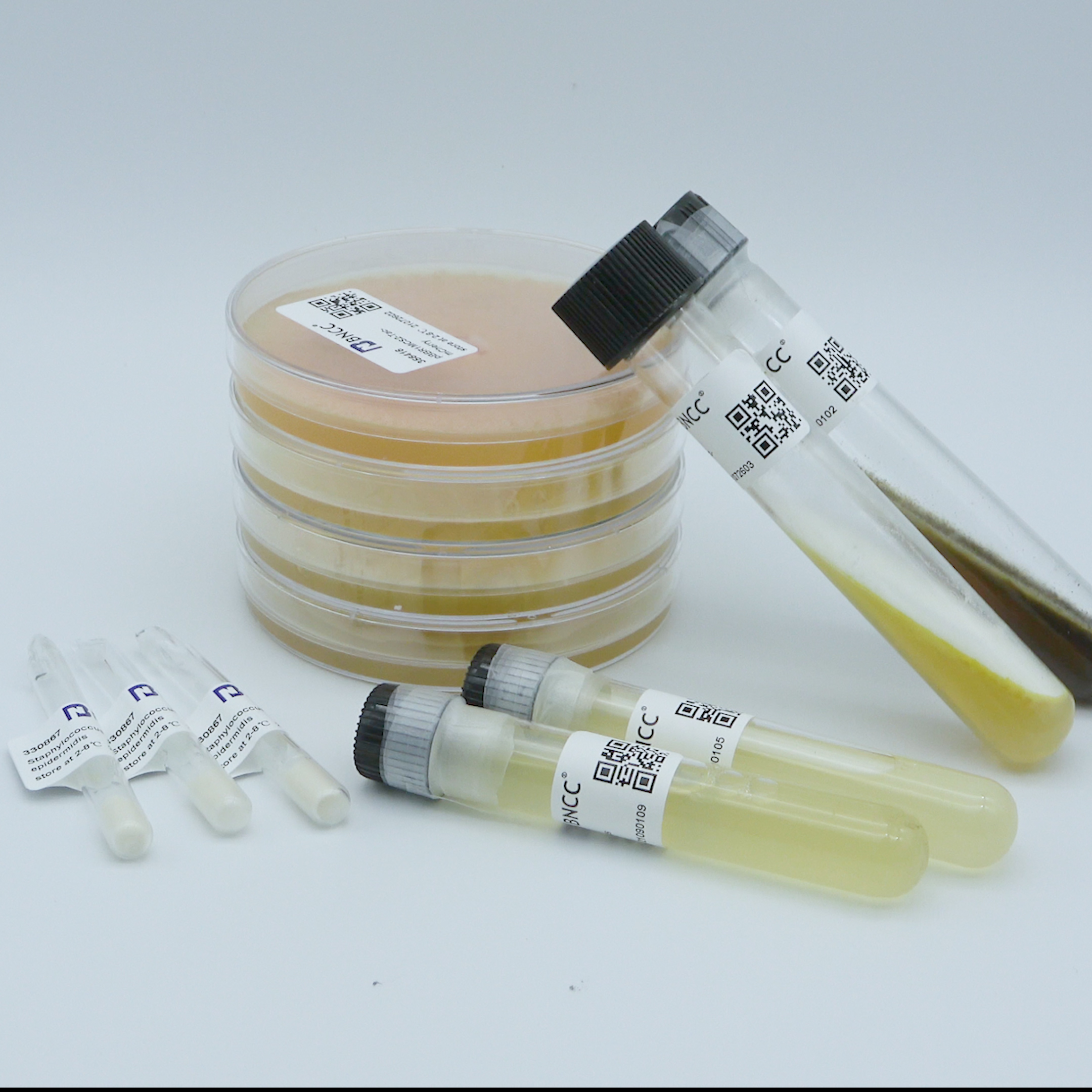
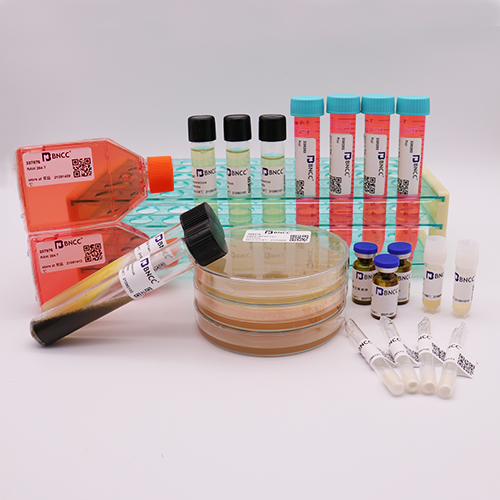
Candida albicans
No. : 353801 / CMCC(F)98001
Product format: quantitative bacteria tablets
Number of live bacteria : 1.0-1.5x10 3CFU/bottle
Product batch : 191218
Valid until : 2020.12.17
Date of inspection : 2019.12.20
Sampling ratio : ≥2%
Report number : RS19353801-1
Biosafety level :1 , handle in ultra-clean table or safety cabinet
| item |
description |
test results |
| stain microscopy |
gram-negative and positive, cell morphology observed under microscope |
G + yeast, spherical |
| colony morphology |
observation of single colony morphology |
bright white, moist, raised, with neat edges |
| purity |
non-selective medium culture, colony observation and microscopic examination |
No bacteria |
| Reference value of live bacteria |
non-selective medium plate, live bacteria count |
1120CFU/bottle |
| phenotype and genotype analysis |
phenotypic testing, gene sequencing |
qualified |
| test conclusion |
after quality inspection by BNCC quality control testing system, the product is qualified |
|
Product list :1.0-1. 5x103CFU quantitative strain 1 bottle, 1mL dissolved solution 1 bottle, strain certificate 1 copy;
Validity period : unopened, below -20 ℃, 12 months;
Handling procedure:
1 Open the penicillin-bottle according to the arrow indication on the vial cap;
2 Transfer the strain tablets into the vial of solution, capped with rubber plug, and shake to dissolve
3 It will be 1.0-1.5x10 3cfu/ml after full dissolved, and use appropriate amount according to the operation demand
4 The bacterial tablets shall be used up once and shall not be retained once the penicillin bottled is opened.
Notes:
1 This product is a quantitative strain. Improper storage conditions and handling procedure will affect the accuracy of the strain quantities. Therefore, please operate in strict accordance with the above mentioned instructions.
2 Waste generated from the handling process should be discarded after high-pressure sterilization

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese