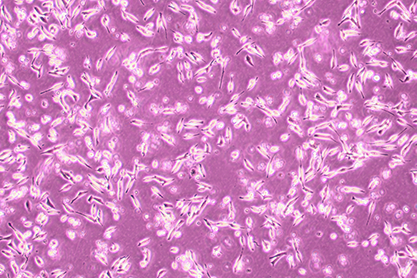
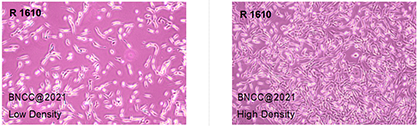
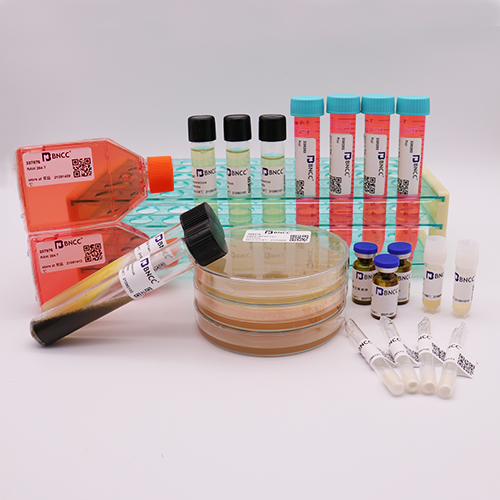
1. Name:R 1610
2.No.:340923
& nbsp; 3. Growth properties : ■ adherent □ suspension □ semi-suspended and semi-adherent
4. Growth conditions:
| culture medium |
90% & nbsp; High sugar DMEM |
| serum |
10% FBS (Diagnovum ) |
| temperature |
37 ℃ |
| air condition |
5% CO2,95% AIR |
| frozen storage conditions |
culture medium 50%, serum 40%, DMSO 10% |
5. Composition:
| composition |
specifications |
| a bottle of cells |
T25 |
| cell culture and operating instructions |
1 copy |
Receiving notice:
1 Upon arrival, it is suggested to sit the cells in a incubator for about 4 hours, and then renew the media for recovery or subculture according to the cell density.
2 If the adherent cells are received in the form of ( partial) suspension, please centrifuge the suspended cells in time, add 15% serum complete medium to a fresh culture dish / vial and continue to culture for 3 days; At the same time, the remaining adherent cells in the original culture flask were renewed with 15% serum complete medium and cultured for 2-3 days. If the cells do not proliferate after 3 days but continue to detach and die, please contact the technicians.
3 Slow growth of adherent cells: properly increase the serum concentration (no more than 20%) or transfer to a fresh culture flask for further culture after trypsin digestion according to the cell density.
4 Uneven growth: if adherent cells grow unevenly and is island-like, they can be digested, dispersed, and cultured with fresh medium.
Recovery and subculture procedure ( under strict aseptic conditions )
1 Remove the medium in the original culture flask, rinse twice with PBS, and add 1~2 ml of 0.25% EDTA for trypsin digestion (usually in 1~2min)
2 Observe the digestion under the microscope. When the cell edge shrinks and adherent is loose (but not floating), remove the trypsin, add 6~8ml complete medium, aspirate the cell layer off.
3 Transfer part of the cell suspension to a fresh culture vessel / flask, add appropriate complete medium, and culture it in the incubator.
4 Pay attention to the change of pH value of medium and cell density, renew the medium regularly, and repeat the subculture or cryopreservation when the cell density reaches 70%-80%.
Special attention: (if handle in public laboratory or first time to culture the cells, it is recommended to add penicillin streptomycin into the media)
1 Upon the receipt of the cells, renew the solution with fresh 10% FBS medium as soon as possible. It is not recommended to use the medium used for transportation in the original bottle.
2 Please take photos and contact the technicians in time if the culture flask leaks upon the receipt of cells.
3 Any compliant on the cells, please take photos and contctat our technicians.

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese