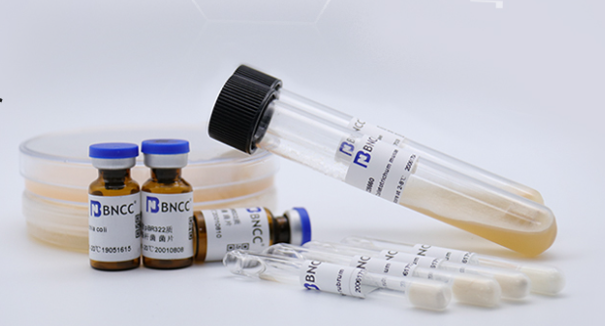
Severe epidemic situation, detection upgrade丨BNCC pathogenic nucleic acid detection standard products made another breakthrough.
Published:2023-01-15 10:01
Editor:BNCC
n response to the needs of the new coronavirus epidemic prevention, all localities have focused on strengthening the construction of nucleic acid testing capabilities, and the amount of nucleic acid testing has increased significantly. The National Health and Medical Commission has issued a series of notices to strengthen the quality management of laboratories, emphasizing that medical institutions should strengthen the quality control of nucleic acid testing, and BNCC has supplemented relevant pathogen nucleic acid testing standards based on the market—Vibrio parahaemolyticus nucleic acid testing standards Standard products, Salmonella Enteritidis nucleic acid detection standard, Bacillus pertussis nucleic acid detection standard, Staphylococcus aureus nucleic acid detection standard and other standard products, through the cultivation of related bacteria, the cultured bacteria are subjected to nucleic acid extraction, purification, identification, and digital PCR Absolute quantification of the concentration of nucleic acids is performed.
This issue introduces related products by taking the standard product of Vibrio parahaemolyticus nucleic acid detection as an example. This standard product is not infectious, has no biosafety risk, and is suitable for the laboratory nucleic acid detection capabilities (sensitivity test and specificity test). Assessment and verification; indoor quality control can be carried out on the relevant laboratories for nucleic acid detection of Vibrio parahaemolyticus, effectively monitoring the status of instruments, personnel operations, and the effectiveness of kits, etc., to ensure the accuracy and reliability of test results.
| Product code |
Product name |
Specifiction |
| BNCC362408 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Freeze-dried;(3.0±2.2)x10^2copies/mL;5vail |
| BNCC362409 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Freeze-dried;(5.0±1.1)x10^3copies/mL;5vail |
| BNCC362410 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Freeze-dried;(4.5±0.5)x10^4copies/mL;5vail |
| BNCC362411 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Freeze-dried;(4.6±0.5)x10^5copies/mL;5vail |
| BNCC362414 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Freeze-dried;(4.2±0.5)x10^6copies/mL;5vail |
| BNCC362415 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Frozen vial;(4.2±2.2)x10^2copies/mL;5vail |
| BNCC362416 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Frozen vial;(4.4±1.1)x10^3copies/mL;5vail |
| BNCC362417 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Frozen vial;(4.3±0.5)x10^4copies/mL;5vail |
| BNCC362418 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Frozen vial;(4.8±0.5)x10^5copies/mL;5vail |
| BNCC362419 |
Vibrio parahaemolyticus nucleic acid detection standard product |
Frozen vial;(4.3±0.5)x10^6copies/mL;5vail |
Instructions: This standard product is available in the form of freeze-dried and frozen.
Freeze-dried form: the standard product should be taken out of the low temperature environment before use, and equilibrated to room temperature; please centrifuge (12000rmp, 60s) before opening the cover; add 100μL enzyme-free sterile water to dissolve after opening the cover, shake evenly, and centrifuge instantly used later.
Frozen: when using this standard product, move it to room temperature until it is completely dissolved, shake evenly, and use it after instantaneous centrifugation. Try to avoid repeated freezing and thawing.
Notice:
1 After receiving the goods, please keep them properly. In case of damage, please contact us within 24 hours.
2 Please operate in a biological safety cabinet in a sterile environment to avoid repeated freezing and thawing.
3 When using the standard, attention should be paid to biosafety protection, and it should be treated as potentially bioinfectious samples. The operation and treatment should comply with relevant regulations.

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese