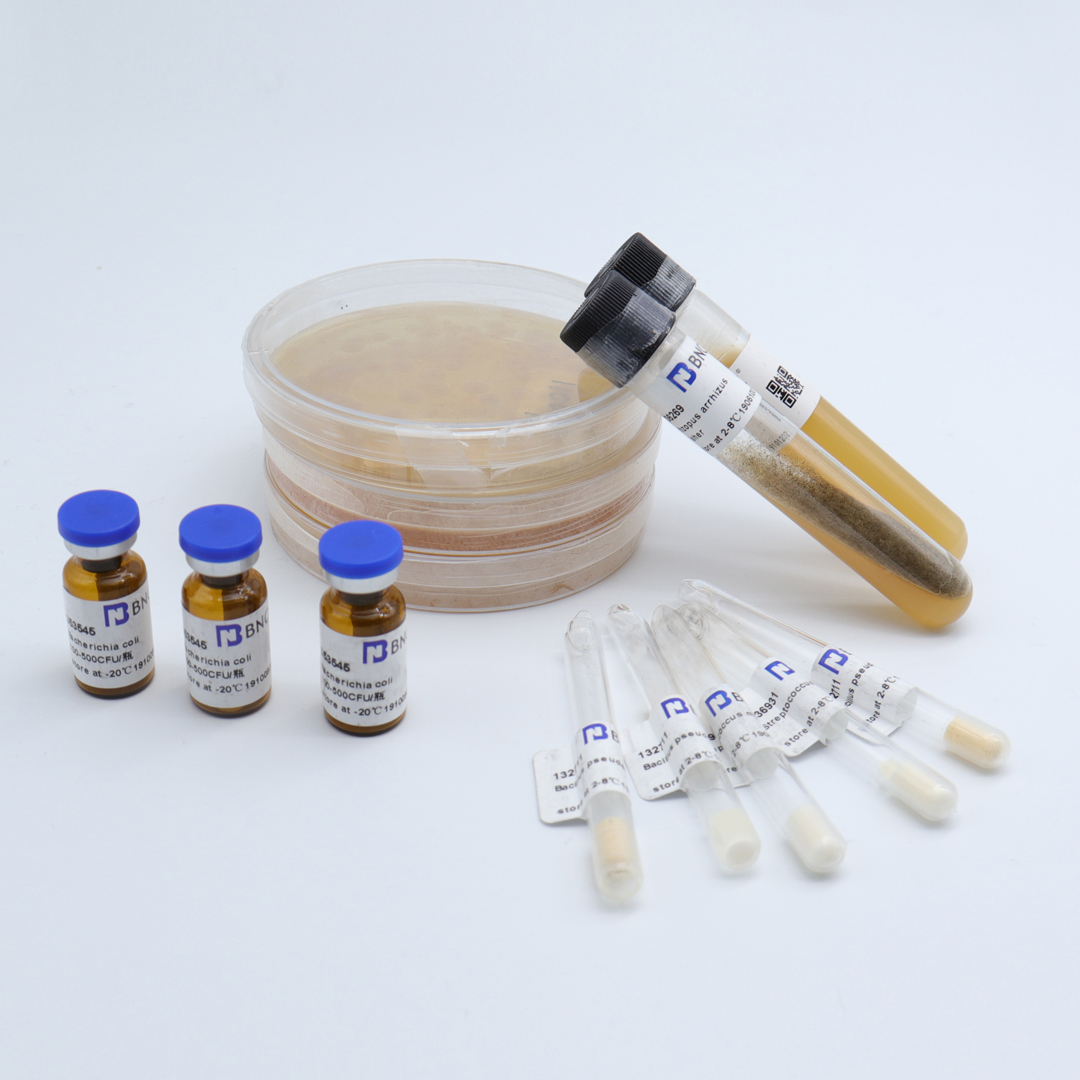
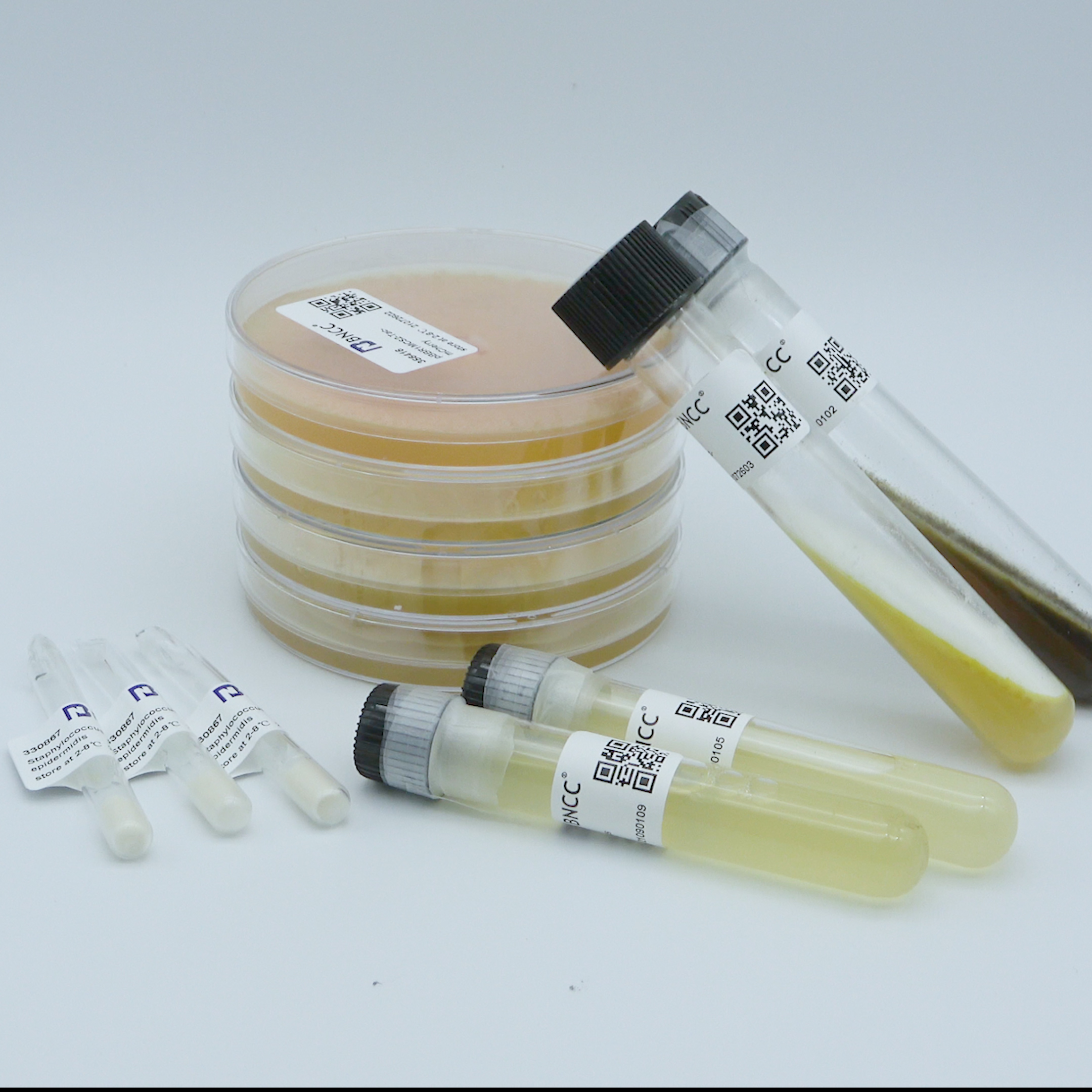
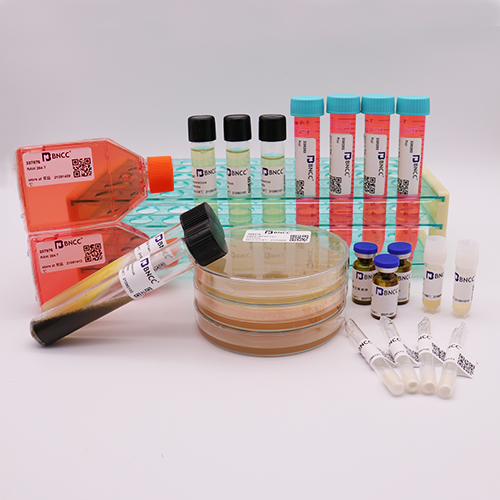
Pseudomonas aeruginosa
Strain number: BNCC 125486 / CMCC10104
Product format: quantitative bacteria tablets 50- 100CFU、bottle
Product batch:22011201
Date of inspection:2022.01.13
Valid until:2023.01.11
Number of viable bacteria:(75±25)CFU/bottle
Sampling ratio:≥2%
Report number: RS22125486-1
Biosafety level: 2, handle in ultra-clear table
| Items |
test results |
description |
| stain microscopy |
gram negative and positive, cell morphology observed under microscope |
G + bacilli |
| colony morphology |
observation of single colony morphology |
light yellow, moist, raised, with neat edges |
| purity |
non-selective medium culture, colony observation and microscopic examination |
No bacteria |
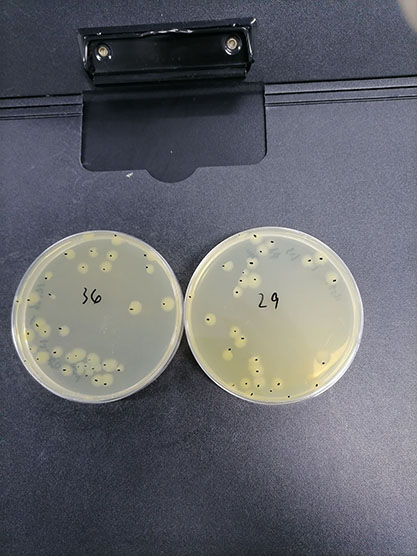
| Reference value of live bacteria |
Non-selective medium plate, viable count |
63CFU/bottle |
| Phenotypic and Genotypic Analysis |
phenotypic testing, gene sequencing |
qualified |
| test conclusion |
after quality inspection by BNCC quality control testing system, the product is qualified |
|
Product list: (75±25)CFU/bottle ,1 bottle of 50-100CFU quantitative strain, 1 bottle of 1mL lysate, 1 strain certificate;
Validity period: unopened, below -20 ℃, 12 months;
Usage:
1. Open the strain and dissolved solution according to the arrow of Xilin bottle cap;
2. Pour the bacteria tablets into the dissolved liquid, cover the rubber stopper, shake and dissolve;
3. After fully dissolved, it is (75±25)CFU/mL, absorbed in proportion according to the test requirements;
4. Please use up immediately after opening, and do not store it;
Notes:
① This product is a quantitative strain. Improper storage conditions and use methods will affect the accuracy of the number of strains, so please operate in strict accordance with the above storage conditions and methods.
②Used germ-carrying consumables should be discarded after autoclaving.

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese