BNCC invites you to participate in the BIOHK 2024 exhibition
Published:2024-09-11 17:18
Editor:BNCC
BNCC attended the BIOHK 2024 exhibition from September 11th to 14th and the booth number is 3E-E03, Wan Chai Convention and Exhibition Center, Hong Kong. BIOHK 2024 is an event not to be missed in the biotechnology industry! BNCC is taking advantage of the momentum to empower the industry and promote development. Welcome all new and old friends to visit our booth and discuss cooperation!

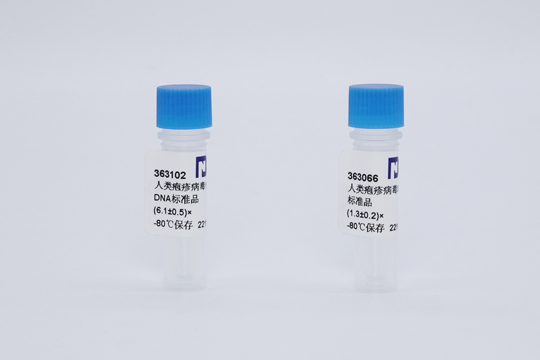
At this exhibition, BNCC displayed more than 100 hot-selling products such as experimental cells, microbial strains, microbial quality control products, national first-level biomatrix reference materials, organic/inorganic second-level national standard materials, etc., attracting many of the excellent talents and companies. BNCC prepared carefully and was full of sincerity, just to meet you!
Hong Kong International Biotechnology Exhibition
Organizer: Hong Kong Biotechnology Association (HKBIO)
Date: September 11 to 14, 2024 (from Wednesday to Saturday)
Address: Wan Chai Convention and Exhibition Center, Hong Kong

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese