Five kinds of diarrhea-causing Escherichia coli nucleic acid detection kit certificates
1. Product information
Sample name: five kinds of diarrhea causing Escherichia coli nucleic acid detection kit (multiplex fluorescence PCR method)
Sample number: 361407
Sample batch: 220428
2. Product features
| Reagent composition |
reaction liquid a tube, reaction liquid B tube, positive quality control product, negative quality control product |
| traceability |
BNCC186732 enteropathogenic Escherichia coli, BNCC345676 enterohemorrhagic Escherichia coli, BNCC340975 enteroinvasive Escherichia coli,
BNCC195617 enterotoxigenic Escherichia coli, BNCC340663 intestinal agglomeration Escherichia coli, ATCC25922 Escherichia coli
|
| save and transport |
-20 ℃, valid for 1 year, dry ice transportation |
| applicable fields |
This test kit is suitable for enteropathogenic Escherichia coli (EPEC) in water-like feces, suspected contaminated water, food and other samples,
intestinal hemorrhagic Escherichia coli (EHEC)/Shiga toxin-producing Escherichia coli (STEC), intestinal invasive Escherichia coli (EIEC),
The pathogenic genes of enterotoxigenic Escherichia coli (ETEC) and intestinal agglomeration Escherichia coli (EAEC) were tested to determine the five types of diarrhea-causing Escherichia coli infection.
|
| *Note: If you have any questions about this kit, please contact our center (BNCC) for help before use |
3. Instruction
1. Experimental preparation: Dissolve the various components in the kit on ice before use, mix gently and centrifuge briefly, and place on ice for later use;
2. Sample processing: Prepare the sample according to 《GB4789.6-2016 Diarrhea-causing Escherichia coli Detection》;
3. Reagent preparation: experimental reaction system (25 μL), 21 μL qPCR mixed reaction solution, 4 μL template;
4. Reaction conditions: 95°C 3min 1cycle; 94°C 10s, 50°C 30s 40cycle;
4. Product performance indicators
1. Minimum detection limit: 1.0×103copies/mL.
2. Linear range: 1000~2×1010copies/mL.
3. Cross-reactivity: against other pathogens (Salmonella, Vibrio cholerae, Vibrio parahaemolyticus, Pseudomonas aeruginosa, Staphylococcus aureus, Campylobacter jejuni, Legionella spp. , ) were not cross-reactive.
4. Precision: The coefficient of variation of the detection precision reference material is less than 5%.
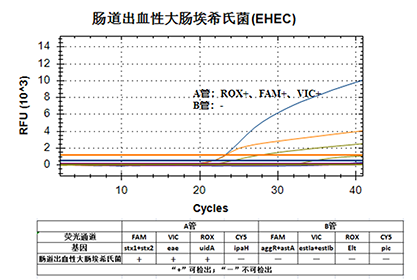
5. Fluorescent channel
This kit uses multiplex real-time fluorescent PCR technology to detect five types of diarrhea-causing Escherichia coli nucleic acids (EPEC, EHEC/STEC, ETEC, EIEC, EAEC) using two PCR reactions A and B on the same sample. Tube A can be used for EPEC, EHEC/STEC, EIEC detection and E. coli species identification, tube B can be used for ETEC and EAEC detection. By collecting the fluorescent signal generated by PCR amplification, it is determined whether the sample to be tested contains nucleic acid of Escherichia coli, and at the same time, its pathogenic type is determined.
| |
|
tube A |
tube B |
| fluorescent channel |
|
FAM |
VIC |
ROX |
CY5 |
FAM |
VIC |
ROX |
CY5 |
| type |
|
Stx1+stx2 |
eae |
uidA |
ipaH |
aggR+astA |
estIa+estIb |
Elt |
pic |
| EPEC |
|
- |
VIC+ |
ROX+ |
- |
- |
- |
- |
- |
| EHEC/STEC |
① |
FAM+ |
- |
ROX+ |
- |
- |
- |
- |
- |
| ② |
FAM+ |
VIC+ |
ROX+ |
- |
- |
- |
- |
- |
| EIEC |
- |
- |
ROX+ |
CY5+ |
- |
- |
- |
- |
| ETEC |
① |
- |
- |
ROX+ |
|
- |
VIC+ |
ROX+ |
|
| ② |
- |
- |
ROX+ |
|
- |
VIC+ |
- |
|
| ③ |
- |
- |
ROX+ |
|
- |
- |
ROX+ |
|
| EAEC |
① |
- |
- |
ROX+ |
- |
FAM+ |
- |
- |
CY5+ |
| ② |
- |
- |
ROX+ |
- |
FAM+ |
- |
- |
- |
| ③ |
- |
- |
ROX+ |
- |
- |
- |
- |
CY5+ |
| non-diarrhea Escherichia coli |
- |
- |
ROX+ |
- |
- |
- |
- |
- |
6. Result judgment
1. The sample has a typical amplification curve, and the Ct value is less than or equal to 35;
2. The sample has no amplification curve, and the Ct value is ≥40 or not detected;
3. If the t value of the sample is >35 and <40, it needs to be re-measured. The retest results were negative if Ct value ≥ 40, and positive if Ct value < 40.
7. Preservation and transportation
1. Dissolve the various components in the kit on ice before use, mix thoroughly and centrifuge briefly and place on ice for later use;
2. In order to ensure the sensitivity of the detection, it is recommended that the qPCR mixed reaction solution be frozen and thawed no more than 3 times;
3. Sample processing, reagent preparation, and sample addition should be carried out in different areas to avoid cross-contamination;
Henan Engineering Research Center of Industrial Microbiology
Website: www.bncc .org .cn Tel: 400-6699-833

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese