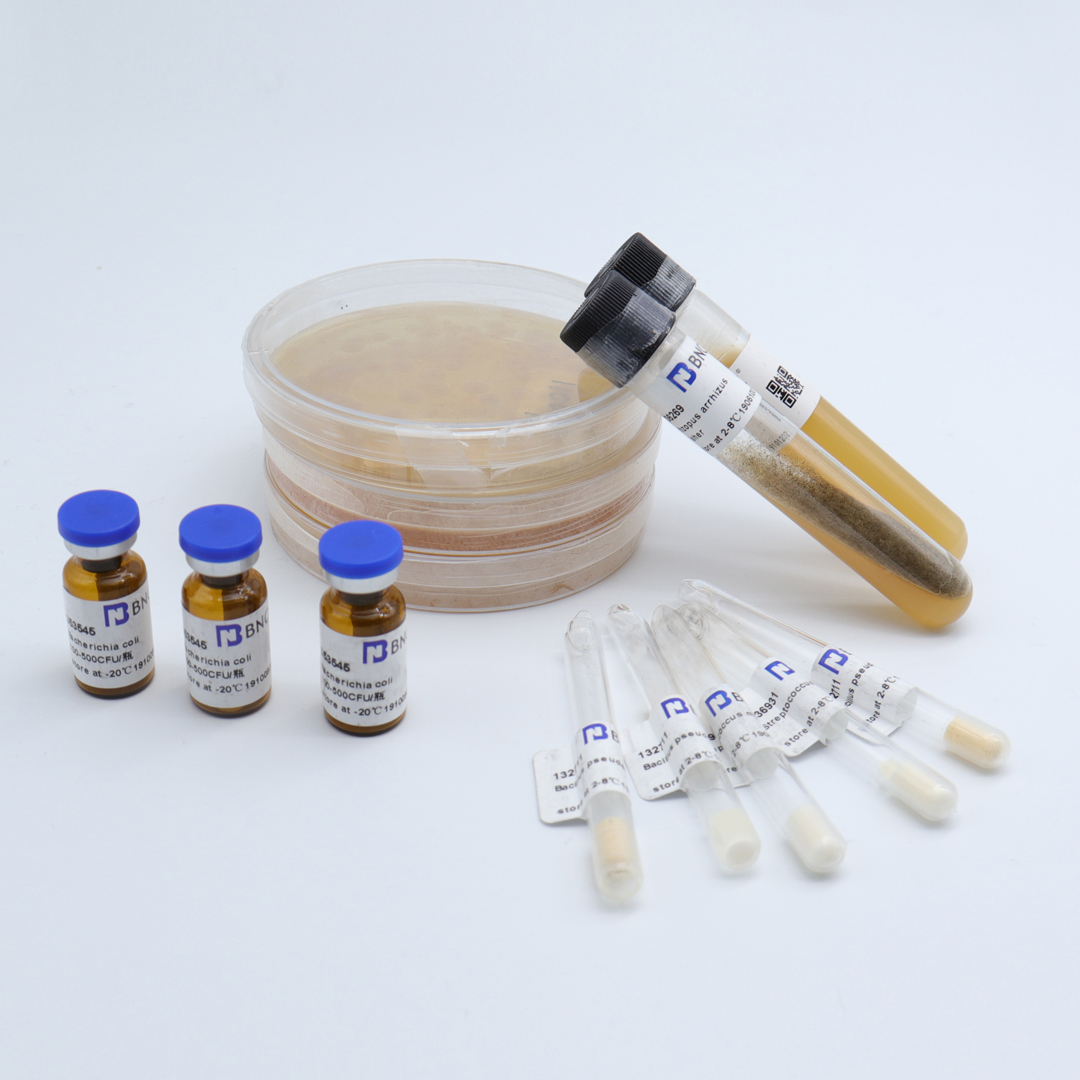
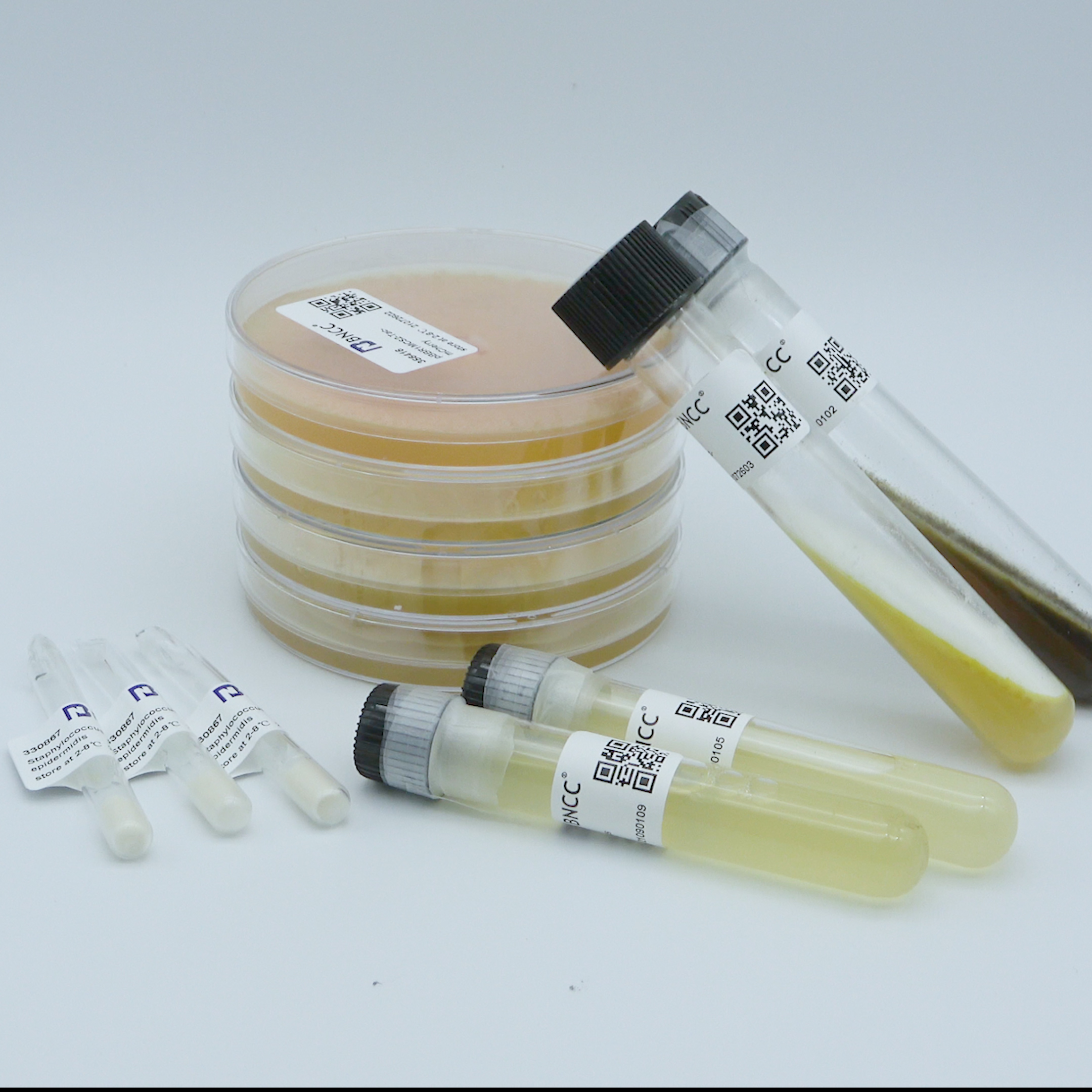
Escherichia coli
Strain number:BNCC 354966 / CMCC 44102
Name: Escherichia coli
Product format: quantitative bacteria tablets Number of viable bacteria: ( 1.8±0.2)×10^6CFU/bottle
Product batch: 220317 Valid until: 2024.03. 16
Inspection date: 2022.03.18 Sampling ratio: ≥2%
Report number: RS22354966-1 Biosafety level: 1, handle in ultra-clear table or safety cabinet
| Item |
Inspection description |
results |
| stain microscopy |
gram negative and positive, cell morphology observed under microscope |
G-bacilli |
| colony morphology |
observation of single colony morphology |
golden yellow, raised, moist, bright |
| Purity |
non-selective medium culture, colony observation and microscopic examination |
No contaminated bacterial |
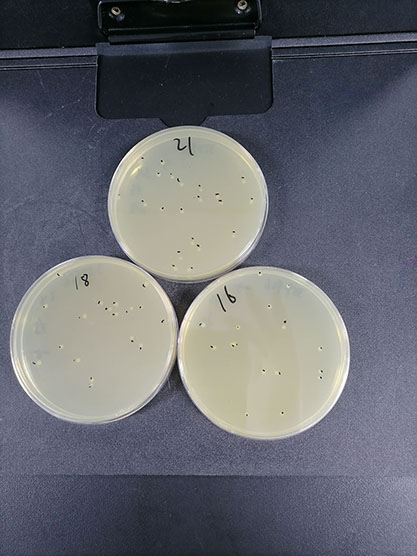
| Reference value of live bacteria |
Non-selective medium plate, viable count |
1.8 × 10^6CFU/bottle |
| Phenotypic and Genotypic Analysis |
phenotypic testing, gene sequencing |
qualified |
| test conclusion |
After the BNCC quality control inspection system quality inspection, the product is qualified |
Product list:One bottle of ( 1.8±0.2)×10^6CFU quantitative strain, one bottle of 1mL dissolution solution and one copy of strain certificate;
Shelf life: unopened, below -20 ℃, 24 months;
Usage:
1. Open the strain and dissolved solution according to the arrow of Xilin bottle cap;
2. Pour the bacteria tablets into the dissolved liquid, cover the rubber stopper, shake and dissolve;
3. after full dissolution, it is (1.8±0.2)×10^6CFU/mL, can be absorbed in proportion according to the test requirements;
4. Please use up immediately after opening, and do not store it;
Notes:
① This product is a quantitative strain. improper storage conditions and usage methods will affect the accuracy of the number of strains, so please strictly follow the above storage conditions and methods.
② Used bacteria-carrying consumables, please autoclave and discard them.

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese