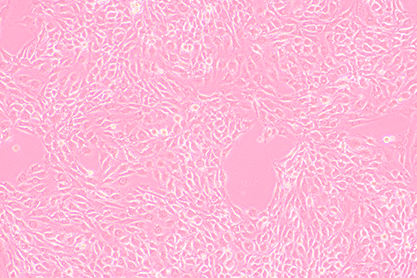
Mouse myeloma cell FO
BNCC number: 360025
Growth characteristics: semi-suspended and semi-adherent growth
Growth conditions: 37℃,5%CO2
Complete medium: 90%DMEM-H+10%FBS
Cryopreservation conditions: 50%DMEM-H+40%FBS+10%DMSO
Receiving notice: if any abnormality is found on the day of receipt, please contact the customer service within 24 hours. If it is overdue, it is deemed that the cells are well. For the frozen cells, it shall be stored in the refrigerator at -80 ℃ upon arrival. If they are not used for a long time, they shall be transferred to liquid nitrogen for storage overnight. T25 format: After receiving the product, let it stand in the incubator for 2-3 hours, and then perform routine operations on the cells. Under sterile conditions, transfer the original bottle of culture medium to a centrifuge tube. After centrifugation at 1000-1200rpm/min for 5-10 minutes, resuspend the cells, transfer the suspended cells to the original T25 flask, add complete medium to 10ml, and culture in an incubator. Please operate in strict accordance with this instruction, otherwise, the reissue service will not be provided in case of cell inactivation.
Recovery steps:
①The frozen vial is taken out of liquid nitrogen or -80 ℃ refrigerator and put into PE gloves, quickly submersed into a 37 ℃ water bath, shaken the frozen vial to accelerate dissolution, and it is advisable to dissolve all within 1min;
② Put the dissolved cell solution into a centrifuge tube containing 9ml of complete culture medium in an ultra-clean table, centrifuge at 1000-1200rpm for 5min, discard the supernatant, resuspend the cells with 1-2ml of complete media.
③ The cell suspension was added to T25 flask containing 5-6ml complete medium and cultured in an incubator.
Subculture:
①Aspirate the culture medium (containing suspended cells) into a centrifuge tube, centrifuge at 1000rpm for 5 minutes, and collect the cells
② Gently pipet the cell layer in the T25 flask with PBS to blow off the cell layer and disperse.
③ dispense the cell suspension into a fresh T25 flask as the ratio of 1:2, add appropriate complete culture medium, make the cell suspension well distributed, and culture in an incubator.
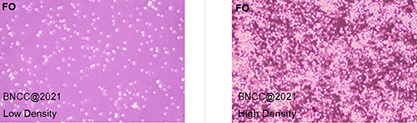
④ Pay attention to the change of pH value of media and cell density, renew the media regularly (2-3 times a week), and repeat the passage operation or cryopreservation when the cell density reaches 80%-90%.
Notes:
①The cells are density dependent. Initiate the subcultivation in T25 flask when the cell density reaches 80%; A subcultivation ratio of 1:2 is recommended.
② If the cells in sealed culture bottle, put it into the incubator for cultivation after handling, with the cap unscrewed.
Recovery record: According to the recovery instructions, the results of the cell recovery are reported as follows:
| Item |
quality standard |
Recovery record |
| viability |
adherence is observed in 18 hours, the cell adherence rate ≥ 80.0% in 12d |
adherence is observed in 18 hours, cell adherence rate ≥ 80.0% in 11d |
| cell morphology: |
lymphoblast-like |
Lymphoblast-like, round |
| attached: |
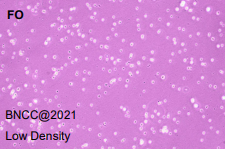 |
 |
| conclusion: good viability, no abnormal cell morphology, qualified |

 info@bncc.com
info@bncc.com
 - English
- English
 - Japanese
- Japanese